Researchers can now detect brand new mutations in individual malaria parasites infecting humans. Such high resolution could help us understand how parasites develop drug resistance and evade immune responses, and suggest potential treatment targets.
SAN ANTONIO (October 11, 2021) – Understanding how malaria parasites evolve after a human is bitten by an infected mosquito is very difficult. There can be billions of individual parasites in a patient’s bloodstream and traditional genetic sequencing techniques can’t identify the raw material for evolution: new mutations.
“If you want to understand if the parasites are related to each other, if they are all from one mosquito or multiple mosquito bites, and what novel mutations are emerging in an infection, then you have to bring it down to the individual genome level,” says Assistant Professor Ian Cheeseman, Ph.D., and Co-lead of the Host-Pathogen Interactions Program at Texas Biomedical Research Institute.
Thanks to a combination of advanced techniques, Cheeseman and his collaborators are now able to sequence the genomes of individual parasites found in the blood of infected patients. Notably, they can now do this even when the infection burden is very low, which can occur during asymptomatic infections. They describe their approach this month in the journal Cell Host & Microbe. Gaining this incredibly detailed view of malaria parasite genetics and evolution is expected to give researchers and drug companies ammunition to develop more effective treatments, vaccines or therapies.
Malaria infects more than 200 million people a year, killing more than 400,000 in 2019 – most of them young children. Of the five malaria parasite species that infect humans, two are the most widespread: Plasmodium falciparum, which is the deadliest; and Plasmodium vivax, which is the leading cause of recurring malaria infections because it can lie dormant in the liver and reemerge later.
“We were really excited to understand how this dormant liver stage might impact genetic variation and evolution in a P. vivax infection,” says co-first paper author Aliou Dia, Ph.D., a postdoctoral researcher in Cheeseman’s lab who is now at the University of Maryland School of Medicine.
The challenge is that when P. vivax does emerge, it only infects very young red blood cells, so parasites are rare in the blood. Analyzing such low levels of infection is the microbiology equivalent of finding a needle in a haystack.
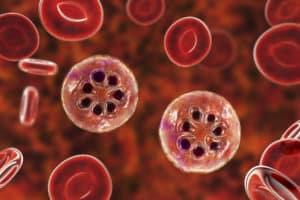
The scientists start with red blood cells, which become slightly magnetic when infected with malaria parasites. They used a high-powered magnet to separate the infected red blood cells from uninfected cells. The infected cells were then run through a machine called a flow cytometer, which uses a laser and fluorescent tags to detect if there is indeed parasite DNA present. Cells with parasite DNA are plopped one by one into test wells and ultimately run through a genetic sequencing machine to decode each individual parasite genome.
Single cell sequencing enables the scientists to precisely compare individual parasite genomes to one another to determine how related they are to each other. They can also really dig down and pinpoint single differences in the genetic code – say an A is changed to a T – to see what happened since the parasite infected that patient.
“We would expect these brand-new mutations to be scattered randomly throughout the genome,” Cheeseman says. “Instead, we find they are often targeting a gene family that controls transcription in malaria.”
But that’s not the only notable thing about the results. What really excites Cheeseman is that when the team compared single cell sequencing data for P. vivax and P. falciparum, the same transcription gene family contained the majority of new mutations for both species.
“We have two different species of malaria from two different parts of the world, Thailand and Malawi,” he says. “When we see the same thing happening independently in different species, this is an example of convergent evolution.”
In other words, similar processes might be shaping similar mutation patterns in both species, even though their last common ancestor was millions of years ago.
The team does not know yet what impact the mutations have on the parasite and its ability to persist and cause damage in human hosts. The mutations may be critical for survival, or something like drug resistance, or may reveal those genes are unimportant.
“We don’t know what these mutations are doing,” Cheeseman says. “But the fact that they are targeting what is seen to be a fairly fundamental part of the parasite lifecycle is interesting and worthy of a lot of follow up.”
###
ABOUT TEXAS BIOMED
Texas Biomed is one of the world’s leading independent biomedical research institutions dedicated to eradicating infection and advancing health worldwide through innovative biomedical research. Texas Biomed partners with researchers and institutions around the world to develop vaccines and therapeutics against viral pathogens causing AIDS, hepatitis, hemorrhagic fever, tuberculosis and parasitic diseases responsible for malaria and schistosomiasis disease. The Institute has programs in host-pathogen interactions, disease intervention and prevention, and population health to understand the links between infectious diseases and other diseases such as aging, cardiovascular disease, diabetes and obesity. For more information on Texas Biomed, go to www.TxBiomed.org.