Researchers at Texas Biomed and University of Pennsylvania have pinpointed how infected cells help or hinder replication and spread of Ebola virus – offering new targets for host-directed therapies designed to block the process. The finding, published in Nature Communications, could also have implications for a wide range of other viruses from HIV to influenza.
SAN ANTONIO (Jan. 16, 2025) — Understanding precisely how Ebola virus hijacks cells and uses them to replicate and spread is critical for developing effective interventions and controlling its highly lethal disease outbreaks. Researchers at Texas Biomedical Research Institute (Texas Biomed) and University of Pennsylvania have made a significant advance in the battle by determining specific ways host cells either assist or block the virus.
The finding, which also opens up significant new areas of investigation for other deadly infectious diseases, involves the Hippo signaling pathway. Named after one of the key proteins involved, the Hippo pathway controls organism and tissue growth by regulating cell proliferation, differentiation, migration and death. The Nature Communications paper is the first to describe interactions between the Hippo pathway and Ebola virus.
The project is a close collaboration between Texas Biomed Assistant Professor Olena Shtanko, Ph.D., and Professor Ronald Harty, Ph.D., at the University of Pennsylvania School of Veterinary Medicine and their labs. The Penn team conceived of the research idea and conducted lower containment experiments, while experiments requiring live Ebola virus were conducted in the biosafety level 4 (BSL-4) lab at Texas Biomed. The Institute is the only independent, nonprofit research center in the nation with a BSL-4 lab equipped to study agents or toxins posing the highest risk for life-threatening infection and disease.
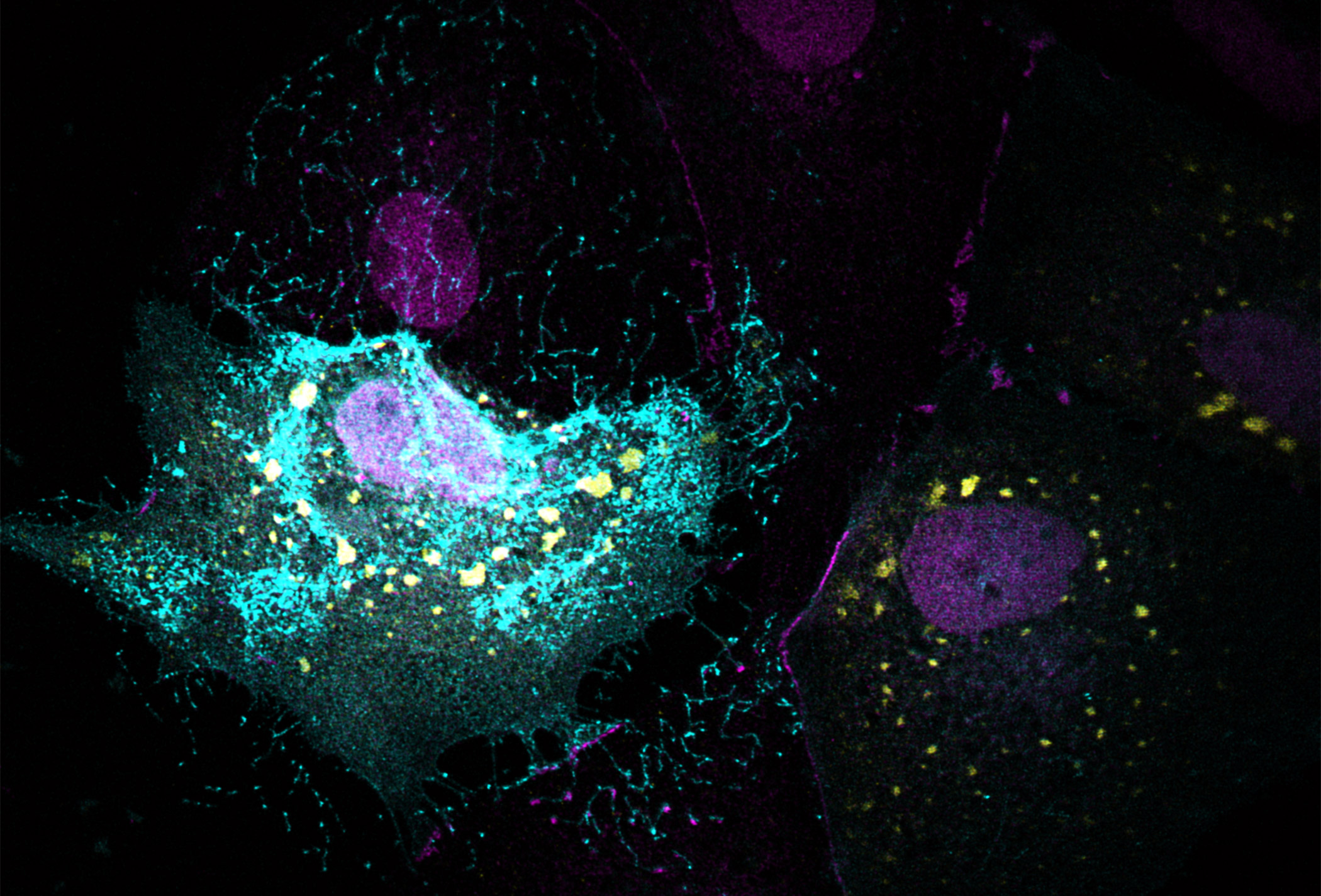
The collaborative research found that two enzymes (LATS1/LATS2) in the Hippo pathway bind to Ebola virus, helping promote transcription of viral RNA. It was further discovered that if another protein in the Hippo pathway, YAP, binds to the enzymes, it helps retain the virus progeny in the cell cytoplasm and ultimately prevents the virus from exiting cells and spreading. In contrast, if the LATS enzymes do not bind to YAP, then YAP migrates to the cell nucleus, where it helps to express cell genes needed for Ebola virus to replicate and spread.
“The Hippo pathway controls so many fundamental processes required for normal cell functioning that it would be highly unlikely for Ebola virus to avoid it,” said Dr. Shtanko. “We suspect that our findings may apply to a range of other viruses as well, such as Lassa virus, Sudan ebolavirus, Marburg virus and HIV.”
She encouraged researchers studying those other viruses to look at the Hippo pathway to see if it is also controlling those viruses’ life cycles.
“If they find similarities, then we can find broad-spectrum treatments that can work against more than one virus,” she said.
Dr. Shtanko will continue to investigate how Ebola virus affects cell behavior, particularly of macrophages – the primary immune cell that the virus infects – and evades detection by the immune system. It is important to identify additional targets for treatments or therapies, since the virus will likely require a multi-pronged approach to contain and eradicate.
Further reading:
Texas Biomed scientists discover new method Ebola virus uses to infect cells – Dec. 2023
Paper:
Liang, J., Djurkovic, M.A., Leavitt, C.G. et al. Hippo signaling pathway regulates Ebola virus transcription and egress. Nat Commun 15, 6953 (2024). https://doi.org/10.1038/s41467-024-51356-z